Protein Assembly
How do proteins and biomolecules assemble in extracellular space?
Understanding vision through lens crystallin proteins. Positioned within the eye, the vertebrate lens supports vision by transmitting and focusing light to the retina. As an adaptive glassy material, the lens comprises densely-packed, polydisperse crystallin proteins that organize to resist aggregation and crystallization at high densities. Despite nearly two centuries of research on this system, little is known about how crystallin proteins coordinate with one another to achieve tissue transparency. We explore the biophysics and chemistry underlying this assembly over multiple spatial scales and describe implications for regulating optical properties.
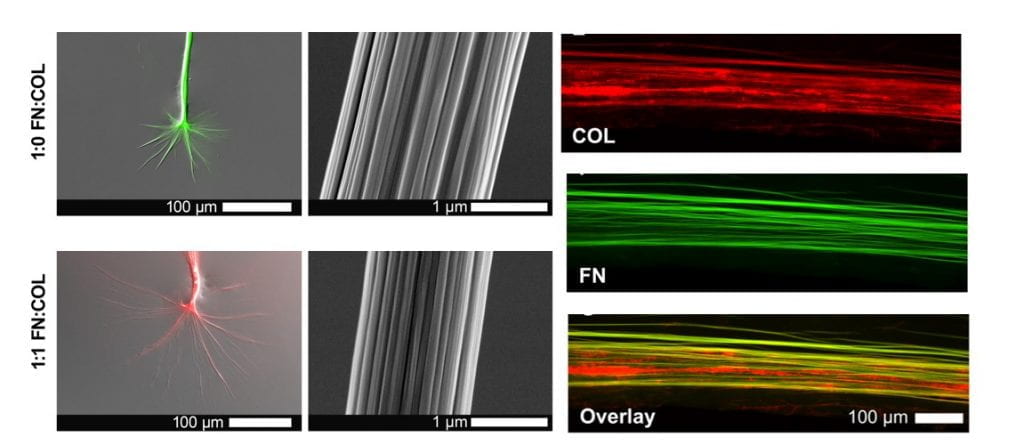
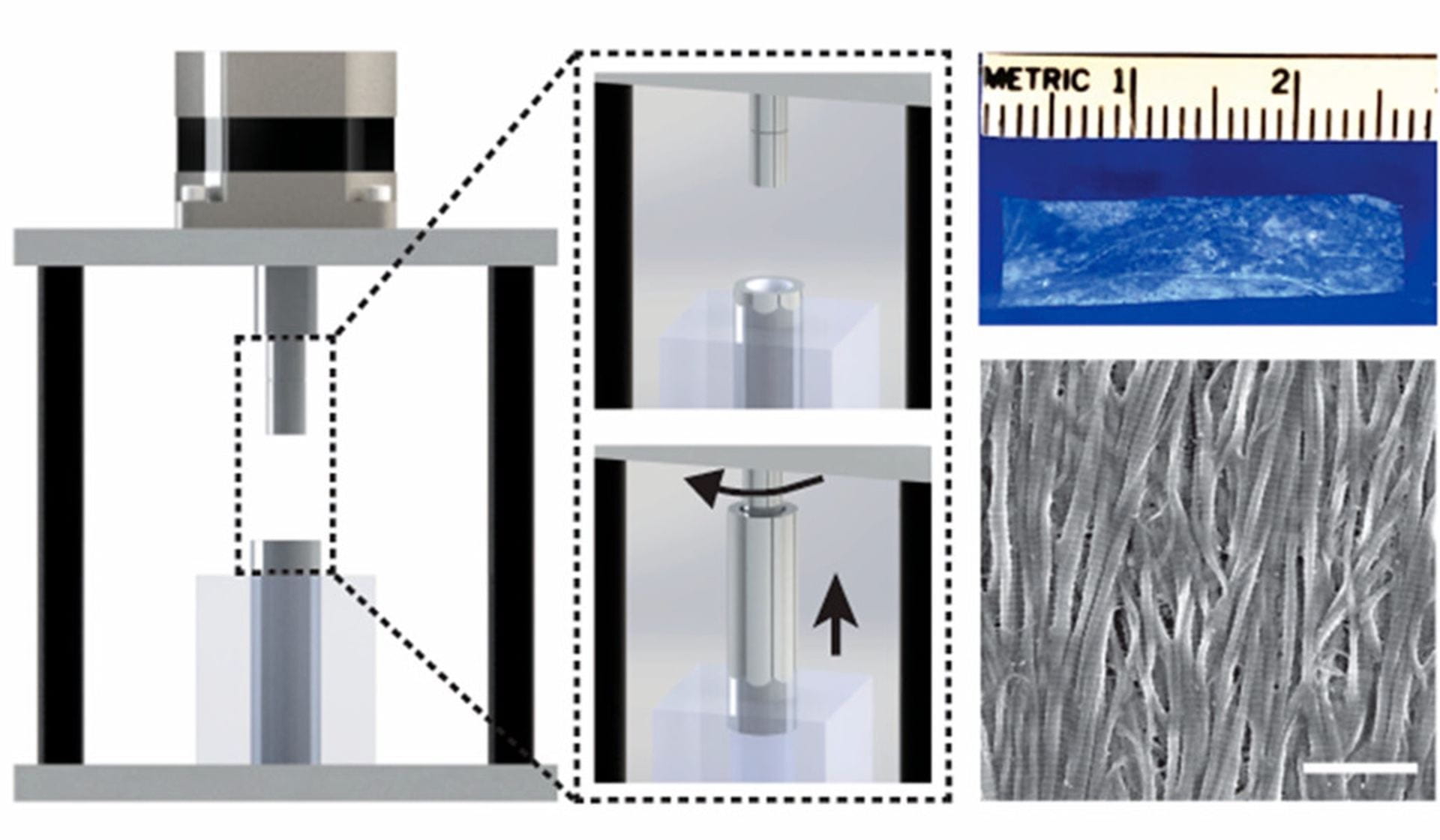
Inspired by how cells build, modify, and reinforce extracellular matrix (ECM) fibers in dynamic living tissues, we develop and test experimental strategies to induce protein fibrillogenesis across multiple spatial scales (nano- to centi- meter). Our focus to date has been on using ECM proteins (collagen and fibronectin) and biomolecules (saccharides) to understand the pivotal events which precede fiber assembly (e.g., conformational unfolding and accelerated nucleation) and growth in the absence of cellular control.
With our advanced understanding of the molecular contributions to fibrillogenesis, we design, build, and test devices for creating synthetic tissue by guiding the rapid alignment and polymerization of protein networks in a variety of fluidic environments. This is achieved using electrically controlled rotating concentric cylinders that create a unique set of fluid dynamics, termed Couette-flow. Our initial synthetic tissue scaffolds currently consist of fibrillar collagen, but preliminary data suggests the immediate potential to incorporate other biomolecules in personalized tissue grafts that we are prototyping with collaborators.